| | | Board Certified In Personal Injury | 28 Years Experience | (903) 531-9300 | | Dear Earl ,Too many new medical devices reach the market without extensive testing or review. Read our newsletter this month to learn more about this important issue and ways you can protect yourself and your family from defective medical devices. | |
| | 28 Years Experience Trying Personal Injury Cases Counts
After 28 years of handling personal injury cases we've learned that sometimes you have to "just say no" to the insurance company and saddle up and go try the case in front of a jury. We did just that in June of this year in a car wreck case involving some pretty serious injuries. The Defendants' auto liability insurance company made a lowball offer and dug in. We said "NO" and headed to the court house. I guess being hard headed has its advantages because a Smith County jury awarded us more than seven times what the insurance company had offered. It was a good day for the good guy. Continue reading.
|
| Faulty Products Put Patients at Risk Some Popular Medical Devices Escape FDA Scrutiny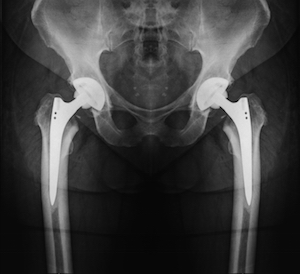 | | Arthroplasty, the reconstruction or replacement of a joint, as shown here in this hip x-ray, is big business for aging baby boomers. |
Countless patients around the world depend on advanced medical devices to improve their quality of life. As the $110 billion medical device industry cranks out thousands of products every year, eye-opening reports continue to surface detailing faulty devices, repeat surgeries and massive recalls. The FDA argues patients need access to life-saving devices quickly. However, a speedy approval process means some risky devices are cleared without clinical testing, and once on the market receive limited oversight. You should know the risks and realities of medical devices before one is put in your body. Fatally flawed approvals. >>> | BY THE NUMBERS / | $133
Billion | The size of the United States medical device market is expected to surpass $133 billion by 2016. At $110 billion, it is currently the largest medical device market in the world. | 71%
Failed | A 2011 study found that 71 percent of Class I medical device recalls (those deemed most likely to cause serious adverse outcomes or death) were cleared through the FDA’s 510(k) fast-track approval process. | 1,190
Recalls | According to a recent FDA report, recalls of defective medical devices nearly doubled from 2003 through 2012: 1,190 in 2012 compared with 604 in 2003. |
| BOOKMARK FAVORITES / |  Routine Surgical Device May Spread Cancer Routine Surgical Device May Spread Cancer
Many surgeons use a device called a morcellator as a way to do noninvasive hysterectomy surgery. Amy Reed, a doctor herself, says the morcellator used in her 2013 hysterectomy spread cancer all over her stomach. View video.  Large Portion of FDA-Approved Devices Lack Safety Evidence Large Portion of FDA-Approved Devices Lack Safety Evidence
A recent study from the Wall Street Journal shows the majority of moderate- to high-risk medical devices approved by the FDA lack scientific evidence to verify safety and effectiveness with patients. View video.  FDA Knew Device Might Expose Patients to Deadly ‘Superbug’ FDA Knew Device Might Expose Patients to Deadly ‘Superbug’
Experts say the FDA has known since at least 2009 that the medical devices involved in a superbug outbreak that infected 179 patients in Los Angeles could transmit lethal infections but did not require more stringent safety standards. View video. |
|